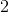All SAT II Chemistry Resources
Example Questions
Example Question #1 :Reactions And Equilibrium
A scientist makes a solution by adding 0.2 grams of
Possible Answers:
Correct answer:
Explanation:





Timothy
Certified Tutor
Certified Tutor
University of Maryland-Baltimore County, Bachelors, Biochemistry and Molecular Biology. Eastern Virginia Medical School, PHD,...
All SAT II Chemistry Resources
Popular Subjects
Chemistry Tutors in Los Angeles,Chemistry Tutors in Denver,Calculus Tutors in Houston,GMAT导师在芝加哥,Math Tutors in Dallas Fort Worth,Math Tutors in Chicago,Physics Tutors in San Francisco-Bay Area,English Tutors in Phoenix,SAT Tutors in Houston,ACT Tutors in Seattle
Popular Courses & Classes
SAT Courses & Classes in San Diego,GMAT Courses & Classes in Chicago,GMAT Courses & Classes in San Francisco-Bay Area,GRE Courses & Classes in Phoenix,ACT Courses & Classes in Seattle,ISEE Courses & Classes in Los Angeles,GRE Courses & Classes in San Francisco-Bay Area,Spanish Courses & Classes in Los Angeles,Spanish Courses & Classes in San Francisco-Bay Area,ISEE Courses & Classes in Washington DC
Popular Test Prep
GMAT Test Prep in Dallas Fort Worth,GMAT Test Prep in Los Angeles,LSAT Test Prep in San Francisco-Bay Area,ACT Test Prep in Seattle,LSAT Test Prep in Phoenix,SSAT Test Prep in Denver,MCAT Test Prep in San Francisco-Bay Area,GMAT Test Prep in San Francisco-Bay Area,SAT Test Prep in Washington DC,LSAT Test Prep in Miami