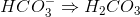All SAT II Chemistry Resources
Example Questions
Example Question #11 :Sat Subject Test In Chemistry
Which of the followingcannotact as a Bronsted-Lowry base (proton recipient) in aqueous solution?



Example Question #1 :Reactions And Equilibrium
A scientist makes a solution by adding 0.2 grams of





Example Question #1 :Reactions And Equilibrium
What is the oxidation number of nitrogen in
First, note that the molecule does not have a charge, meaning that the oxidation numbers of each atom must add up to zero. Hydrogen has an oxidation number of





Example Question #1 :Reaction Types
The following is a modified true/false question. In it, you must decide if each individual statement is true (T) or false (F). If both are true, then you must also decide if the second statement is a correct explanation (CE) of the first statement.
I: An

BECAUSE
II: reduction is a loss of electrons
F, T
F, F
T, T (Statement II is not a correct explanation of Statement I)
T, T, CE
T, F
T, F
Statement II is false: reduction is a gain of electrons. Looking at statement I, the ion's charge would decrease by 3 in becoming a metal, which suggests that the ion gains 3 electrons. Thus, it is undergoing reduction.
Certified Tutor
Certified Tutor
All SAT II Chemistry Resources