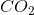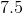一个ll SAT II Chemistry Resources
Example Questions
Example Question #1 :Stoichiometry
When the equation for the reaction shown below is balanced with the lowest whole-number coefficients, what is the sum of the coefficients of the reactants?
___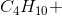


Possible Answers:
Correct answer:
Explanation:
The coefficients of the equation, when balanced, should be 2, 13, 10, and 8. This problem wants to know the sum of thereactants, so only the coefficients on the left side of the reactants should be added. The sum of 2 and 13 is 15, so 15 is the correct answer.
Brianna
Certified Tutor
Certified Tutor
Fairfield University, Bachelor of Science, Biology, General. National University of Ireland at Galway, Master of Science, Neu...
一个ll SAT II Chemistry Resources
Popular Subjects
Chemistry Tutors in Los Angeles,MCAT Tutors in San Francisco-Bay Area,Physics Tutors in Houston,LSAT Tutors in Philadelphia,ISEE Tutors in Dallas Fort Worth,SAT Tutors in Philadelphia,Reading Tutors in Atlanta,Chemistry Tutors in Miami,Statistics Tutors in Los Angeles,Reading Tutors in Denver
Popular Courses & Classes
GMAT Courses & Classes in San Diego,Spanish Courses & Classes in Phoenix,GMAT Courses & Classes in Phoenix,SAT Courses & Classes in Dallas Fort Worth,LSAT Courses & Classes in Philadelphia,一个CT Courses & Classes in Washington DC,Spanish Courses & Classes in Houston,MCAT Courses & Classes in Denver,SSAT Courses & Classes in Los Angeles,MCAT Courses & Classes in Miami
Popular Test Prep
一个CT Test Prep in New York City,一个CT Test Prep in Washington DC,ISEE Test Prep in New York City,LSAT Test Prep in San Francisco-Bay Area,LSAT Test Prep in San Diego,GMAT Test Prep in Atlanta,SAT Test Prep in Chicago,SAT Test Prep in Boston,LSAT Test Prep in Los Angeles,GMAT Test Prep in Phoenix