All Organic Chemistry Resources
Example Questions
Example Question #4 :Carbonyl Reactions
Which of the following results in a single ketone product following acid catalyzed hydration?
5-decyne
None of these answers
2-decyne
3-decyne
4-decyne
5-decyne
在酸催化水合作用,羟基组replaces one of the bonds in the triple bond and a double bond is formed. This is called an enol. The enol naturally turns into a ketone in a process called tautomerization. The hydroxy group can attach to either carbon across the double bond, and naming is done so that substituents have the lowest numbers. Only on 5-decyne will result in a single product, as no matter which carbon the hydroxy group bonds to, it is still on carbon 5. Thus, the only final product is 5-decone.
The other answer options will still react, but will form multiple products due to lack of symmetry.
Example Question #5 :Carbonyl Reactions
What is the product of the reaction shown?

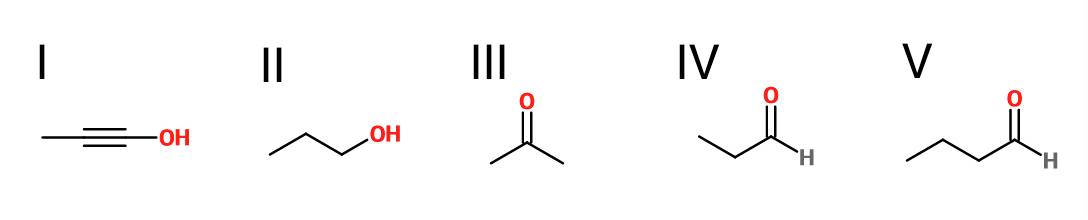
IV
III
V
II
I
IV
First step: bromination across the double bond
Second step: double dehydrohalogenation and removal of terminal alkyne hydrogen
Third step: neutralization of the molecule
Fourth/fifth step: hydroboration/oxidation, followed by keto/enol tautomerization
Certified Tutor
All Organic Chemistry Resources





