All MCAT Biology Resources
Example Questions
Example Question #21 :Isomerism And Stereoisomers
Which answer choice is an enantiomer of the molecule shown below?
An enantiomer is defined as a stereoisomer that is a mirror image of another. In other words, if a molecule was placed in front of a mirror, its mirror image would be the enantiomer. Questions such as these require a bit of visualization, but should be easy points. In addition, if you see the answer choice quickly and are confident in your decision, move onto the next question without spending time on the other choices, as mental geometry and visualization may take more time than anticipated.
Example Question #22 :Isomerism And Stereoisomers
A mystery compound has only one chiral carbon. The enantiomers of this molecule are placed in separate beakers. Which of the following statements is false?
The two configurations will boil at the same temperature
Combining the beakers will result in an optically inactive mixture
The R configuration will bend light clockwise and the S configuration will bend light counter-clockwise
All of these are true
The two configurations will melt at the same temperature
The R configuration will bend light clockwise and the S configuration will bend light counter-clockwise
Enantiomers are defined as compounds that are mirror images of one another. They have the same basic physical and chemical characteristics, but differ in how they bend plane-polarized light. Although we know that the angle is inverted between configurations, R- and S- does not tell us which direction the light will be bent. This information requires further experimentation.
Note that in some cases, enantiomers can demonstrate different properties, such as different levels of toxicity to the body. For this reason, certain drugs are selected for only a single enantiomer.
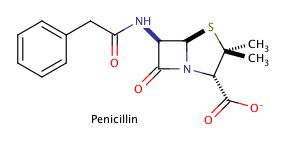
Example Question #51 :Organic Chemistry
Shown above is the chemical structure for penicillin, a common prescription drug. How many chiral carbons does penicillin have?
Five
Zero
Two
Three
One
Three
The correct answer is three. The key to finding chiral carbons is to look for carbons that are attached to four different substituents. We can immediately eliminate any carbons that are involved in double bonds, or that have two hydrogens attached. Given this, we find that there are three chiral carbons. Note that carbon chains of varying content will qualify as different substituents, allowing chiral carbons to bond to two other carbons.
Example Question #23 :Isomerism And Stereoisomers
How many chiral centers does the following molecule contain?
4
3
2
0
1
1
一个手性中心的定义是一个四面体原子with four unique substituents. In the molecule above there exists only one carbon that has four different substituents, which is carbon #4 (counting from the right). Carbon #4 has -chloro and -hydroxy groups, as well as two different longer alkyl chains. No other atom has four unique substituents.
Example Question #24 :Isomerism And Stereoisomers
Ephedrine has the IUPAC name of 2-(methylamino)-1-phenylpropan-1-ol.

The stereochemical assignments for carbons 1 and 2 are __________ and __________, respectively.
R . . . R
The molecule contains only one stereocenter.
S . . . S
R . . . S
S . . . R
R . . . S
We can easily identify the carbons based on the IUPAC name. Carbon 1 will be bound to the phenyl and hydroxy groups, while carbon 2 will be bound to the methyl and amino groups.
For carbon 1 (the carbon attached to the -OH group), the lowest priority group (hydrogen) is already pointing away. The other three groups descend in decreasing priority in a clockwise manner,
For carbon 2 (attached to the -N-CH3group), the stereochemical assignment is S. The lowest priority constituent (hydrogen) is pointing out of the page. The other three groups are in descending priority in a counterclockwise manner,
Example Question #25 :Isomerism And Stereoisomers
An isomer exists such that half of its chiral centers have opposite configurations compared to the original compound. What is the relationship between the isomer and the original compound?
Enantiomer
Geometric isomer
Anomer
Diastereomer
Diastereomer
Absolute configuration tells us the orientation of atoms around a central chiral carbon. Enantiomers are compounds that have the opposite absolute configuration at every chiral carbon in the compound. If only half of the centers are opposite of one another, the two compounds are diastereomers. Diastereomers are isomers in which chiral configuration differs at at least one chiral center, but may differ at several.
Example Question #1 :Intermolecular Forces
Prionsare the suspected cause of a wide variety of neurodegenerative diseases in mammals. According to prevailing theory, prions are infectious particles made only of protein and found in high concentrations in the brains of infected animals. All mammals produce normal prion protein, PrPC, a transmembrane protein whose function remains unclear.
Infectious prions, PrPRes, induce conformational changes in the existing PrPCproteins according to the following reaction:
PrPC+ PrPRes→ PrPRes+ PrPRes
The PrPResis then suspected to accumulate in the nervous tissue of infected patients and cause disease. This model of transmission generates replicated proteins, but does so bypassing the standard model of the central dogma of molecular biology. Transcription and translation apparently do not play a role in this replication process.
This theory is a major departure from previously established biological dogma. A scientist decides to test the protein-only theory of prion propagation. He establishes his experiment as follows:
Homogenized brain matter of infected rabbits is injected into the brains of healthy rabbits, as per the following table:
Rabbit 1 and 2: injected with normal saline on days 1 and 2
The above trials serve as controls.
Rabbit 3 and 4: injected with homogenized brain matter on days 1 and 2
The above trials use unmodified brain matter.
Rabbit 5 and 6: injected with irradiated homogenized brain matter on days 1 and 2
The above trials use brain matter that has been irradiated to destroy nucleic acids in the homogenate.
Rabbit 7 and 8: injected with protein-free centrifuged homogenized brain matter on days 1 and 2
The above trials use brain matter that has been centrifuged to generate a protein-free homogenate and a protein-rich homogenate based on molecular weight.
Rabbit 9 and 10: injected with boiled homogenized brain matter on days 1 and 2
The above trials use brain matter that have been boiled to destroy any bacterial contaminants in the homogenate.
A scientist claims that he has discovered how PrPRes propagates. He claims that the PrPRes interacts with the PrPC by using its own partially negative oxygen atoms to interact with partially positive hydrogen atoms on PrPC. What is true of these bonds?
They are the strongest intermolecular bonds but weaker than covalent bonds.
They are the weakest intermolecular bonds and weaker than covalent bonds.
They are the covalent bonds, forming a disulfide bridge between the two proteins.
They are the weakest intermolecular bonds but stronger than covalent bonds.
They are the strongest intermolecular bonds and stronger than covalent bonds.
They are the strongest intermolecular bonds but weaker than covalent bonds.
Hydrogen bonds are an example of the strongest type of intermolecular bonds. They are, however, still intermolecular, and thus always weaker than covalent bonds.
Example Question #2 :Intermolecular Forces
Cryptosporidium is a genus of gastrointestinal parasite that infects the intestinal epithelium of mammals. Cryptosporidium is water-borne, and is an apicomplexan parasite. This phylum also includesPlasmodium, Babesia,andToxoplasma.
Apicomplexans are unique due to their apicoplast, an apical organelle that helps penetrate mammalian epithelium. In the case of cryptosporidium, there is an interaction between the surface proteins of mammalian epithelial tissue and those of the apical portion of the cryptosporidium infective stage, oroocyst. A scientist is conducting an experiment to test the hypothesis that the oocyst secretes a peptide compound that neutralizes intestinal defense cells. These defense cells are resident in the intestinal epithelium, and defend the tissue by phagocytizing the oocysts.
She sets up the following experiment:
As the neutralizing compound was believed to be secreted by the oocyst, the scientist collected oocysts onto growth media. The oocysts were grown among intestinal epithelial cells, and then the media was collected. The media was then added to another plate whereToxoplasma gondiiwas growing with intestinal epithelial cells. A second plate ofToxoplasma gondiiwas grown with the same type of intestinal epithelium, but no oocyst-sourced media was added.
A scientist is conducting a follow up experiment to the one described above. She is attempting to determine how cryptosporidium adheres to the gastrointestinal mucosa. She determines that the key step is a binding of a surface protein ligand to a receptor. Which of the following forces are common patterns for protein-protein interaction?
I. Hydrogen bonding
II. Coordinate covalent bonding
III. Polar covalent
IV. Metallic bonding
I, II, and III
I and II
I, II, III, and IV
I only
I and IV
I only
Of the choices listed, only hydrogen bonds would be very common among protein-protein bonds. Covalent bonds are strong and permanent, and so are uncommon between macromolecules. Some proteins form disulfide bridges, or covalent bonds between sulfur atoms intra-molecularly, but inter-molecularly covalent interactions are usually not appropriate.
Example Question #3 :Intermolecular Forces
One component of the immune system is the neutrophil, a professional phagocyte that consumes invading cells. The neutrophil is ferried to the site of infection via the blood as pre-neutrophils, or monocytes, ready to differentiate as needed to defend their host.
In order to leave the blood and migrate to the tissues, where infection is active, the monocyte undergoes a process called diapedesis. Diapedesis is a process of extravasation, where the monocyte leaves the circulation by moving in between endothelial cells, enters the tissue, and matures into a neutrophil.
Diapedesis is mediated by a class of proteins called selectins, present on the monocyte membrane and the endothelium. These selectins interact, attract the monocyte to the endothelium, and allow the monocytes to roll along the endothelium until they are able to complete diapedesis by leaving the vasculature and entering the tissues.
下图显示了在bloo单核细胞移动d vessel, "rolling" along the vessel wall, and eventually leaving the vessel to migrate to the site of infection.

Which of the following is likely true about the interactions between selectins and the "rolling" monocytes?
They are likely mediated by intermolecular interactions
They are formed only with the assistance of soluble enzymes
They require ionic charges to mediate the interaction
They are most likely between two surface fatty acid chains
They are likely strengthened by covalent disulfide bridges
They are likely mediated by intermolecular interactions
The interactions that give rise to the neutrophil rolling phenomenon are likely the product of intermolecular bonds, such as hydrogen bonds, that often do not require full ionic charges to be present.
Additionally, proteins and carbohydrates are the typical mediators of these interactions, not fatty acids, and they usually form quickly, reversibly, and spontaneously, without the help of local enzymes.
Example Question #4 :Intermolecular Forces
Type 1 diabetes is a well-understood autoimmune disease. Autoimmune diseases result from an immune system-mediated attack on one’s own body tissues. In normal development, an organ called the thymus introduces immune cells to the body’s normal proteins. This process is callednegative selection, as those immune cells that recognize normal proteins are deleted. If cells evade this process, those that recognize normal proteins enter into circulation, where they can attack body tissues. The thymus is also important for activating T-cells that recognize foreign proteins.
As the figure below shows, immune cells typically originate in the bone marrow. Some immune cells, called T-cells, then go to the thymus for negative selection. Those that survive negative selection, enter into general circulation to fight infection. Other cells, called B-cells, directly enter general circulation from the bone marrow. It is a breakdown in this carefully orchestrated process that leads to autoimmune disease, such as type 1 diabetes.

In the process of negative selection described in the passage, the interaction of T-cells and normal body proteins happens via brief and easily broken biochemical bonds. What type of bonding is most probably involved?
Ionic bonding
Nonpolar covalent bonding
Hydrogen bonding
Coordinate covalent bonding
Polar covalent bonding
Hydrogen bonding
Hydrogen bonding is characteristic of a great deal of the intermolecular interactions seen in biochemical systems. Ionic and covalent bonding are far too permanent, and T-cells would never escape the thymus were these bonding patterns the principal interactions. Ionic and covalent interactions generally represent intramolecular interactions, while intermolecular interactions are more temporary. Common intermolecular forces are hydrogen bonding, dipole interactions, and van der Waals forces. (Note that hydrogen bonding can also be an intramolecular interaction for certain molecular structures).
Certified Tutor
All MCAT Biology Resources