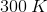All College Chemistry Resources
Example Questions
Example Question #1 :Laws Of Thermodynamics
The second law of thermodynamics states which of the following is true regarding an isolated system?
The entropy can only decrease
The entropy approaches a constant value as the temperature of the system approaches zero Kelvin
The entropy can only increase
Two systems in thermal equilibrium with a third system are in thermal equilibrium with each other
Energy in the form of heat is always conserved
The entropy can only increase
The entropy cannot decrease in an isolated system because the energy can only be degraded. Since the system is isolated, no higher-grade energy—or any energy at all—is being introduced into the system. As a result, the entropy cannot decrease. The other answer choices relate to the other laws of thermodynamics.
Example Question #1 :Thermodynamics
Which of the following statements is true of standard states?
Gas at
Gas at
Solute of
Solute in
Temperature at
Gas at
Standard states are defined as a specific set of conditions, such as when a gas is at


Standard enthalpy of formation, the energy required for form 1 mole of a compound from its constituent elements, occurs when elements are in their standard states.
Example Question #1 :Laws Of Thermodynamics
How much heat is required to raise the temperature of




Example Question #2 :Thermodynamics And Kinetics
How much heat is required to raise the temperature of


Example Question #1 :Thermodynamics And Kinetics
"In a natural thermodynamic process, the sum of the entropies of the interacting systems increases." Which law of thermodynamics does this statement refer to?
First law of thermodynamics
Second law of thermodynamics
Third law of thermodynamics
Zeroth law of thermodynamics
Second law of thermodynamics
There are four main laws of thermodynamics, which describe how temperature, energy, and entropy behave under various circumstances. The zeroth law of thermodynamics helps to define temperature; it states that if two systems are each in thermal equilibrium with a third system, they must be in thermal equilibrium with each other. The first law of thermodynamics negates the possibility of perpetual motion; it states that when energy passes into or out of a system, the system's internal energy changes in accord with the law of conservation of energy. The second law of thermodynamics also negates the possibility of perpetual motion; it states that in a natural thermodynamic process, the sum of the entropies of the interacting systems increases. Lastly, the third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature nears absolute zero.
Example Question #2 :Laws Of Thermodynamics
Calculating heat
How much heat is absorbed by a copper penny as it warms from




Use the equation that relates heat, mass, specific heat, and change in temperature:
Example Question #1 :Thermodynamics
How do we know if a reaction is spontaneous?
The sign of
The second law of thermodynamics
The first law of thermodynamics
The sign of
The value of
The sign of
A reaction is spontaneous if and only if

Example Question #1 :College Chemistry
What is the standard change in free energy for a reaction run at

In this question, we're told of a reaction that is run at a given temperature and with a certain equilibrium constant. We're asked to evaluate the standard change in free energy for this reaction.
First, right off the bat, we can see that the equilibrium constant for the reaction is greater than one. As a result, we know that under standard conditions, equilibrium of this reaction will favor products. Hence, we know right away that
To actually calculate the value of
If we plug in the values that we know, we can solve for the correct answer.
Example Question #2 :Thermodynamics And Kinetics
Calculate the change in free energy, in kilojoules, of the reaction at


记得如何寻找一个自由能的变化reaction from using free energies of formation:
Where
Plug in the given values to find the change in free energy for the reaction.
Example Question #2 :Thermodynamics
Suppose that a given chemical reaction has an enthalpy change of

For this question, we're asked to determine the temperature needed for a reaction to be in a state of equilibrium. We are also provided with the entropy and enthalpy changes for this reaction.
Remember that for a reaction to be in a state of equilibrium, the value for its change in free energy must be equal to zero. Furthermore, we can state the relationship between the change in free energy with the change in entropy, the change in enthalpy, and the temperature as follows.
Because the
Now that we have this expression, we just need to plug in the two values given to us in the question stem. Remember to make the units match though!
Certified Tutor
Certified Tutor
All College Chemistry Resources