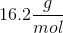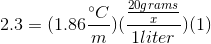All Physical Chemistry Resources
Example Questions
Example Question #2 :Colligative Properties
When 20 grams of a solute is added to a liter of water, the freezing point becomes
Assume that the solute does not make ions in solution.
Possible Answers:
Correct answer:
Explanation:
We can use the freezing point depression equation in order to determine the molar mass of the unknown solute.
Partha
Certified Tutor
Certified Tutor
Gauhati University, Bachelor of Chemistry, Organic Chemistry. Ariel University, Doctor of Philosophy, Organic Chemistry.
All Physical Chemistry Resources
Physical Chemistry Tutoring in Top Cities:
Atlanta Physical Chemistry Tutoring,Austin Physical Chemistry Tutoring,Boston Physical Chemistry Tutoring,Chicago Physical Chemistry Tutoring,Dallas Fort Worth Physical Chemistry Tutoring,Denver Physical Chemistry Tutoring,Houston Physical Chemistry Tutoring,Kansas City Physical Chemistry Tutoring,Los Angeles Physical Chemistry Tutoring,Miami Physical Chemistry Tutoring,New York City Physical Chemistry Tutoring,Philadelphia Physical Chemistry Tutoring,Phoenix Physical Chemistry Tutoring,San Diego Physical Chemistry Tutoring,San Francisco-Bay Area Physical Chemistry Tutoring,西雅图Physical Chemistry Tutoring,St. Louis Physical Chemistry Tutoring,Tucson Physical Chemistry Tutoring,Washington DC Physical Chemistry Tutoring
Physical Chemistry Tutors in Top Cities:
Atlanta Physical Chemistry Tutors,Austin Physical Chemistry Tutors,Boston Physical Chemistry Tutors,Chicago Physical Chemistry Tutors,Dallas Fort Worth Physical Chemistry Tutors,Denver Physical Chemistry Tutors,Houston Physical Chemistry Tutors,Kansas City Physical Chemistry Tutors,Los Angeles Physical Chemistry Tutors,Miami Physical Chemistry Tutors,New York City Physical Chemistry Tutors,Philadelphia Physical Chemistry Tutors,Phoenix Physical Chemistry Tutors,San Diego Physical Chemistry Tutors,San Francisco-Bay Area Physical Chemistry Tutors,西雅图Physical Chemistry Tutors,St. Louis Physical Chemistry Tutors,Tucson Physical Chemistry Tutors,Washington DC Physical Chemistry Tutors
Popular Courses & Classes
SAT Courses & Classes in Los Angeles,MCAT Courses & Classes in Denver,SSAT Courses & Classes in Washington DC,SSAT Courses & Classes in Dallas Fort Worth,LSAT Courses & Classes in Miami,GMAT Courses & Classes in Atlanta,LSAT Courses & Classes in Philadelphia,MCAT Courses & Classes in Seattle,Spanish Courses & Classes in Boston,西班牙语课程&类在纽约市