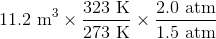All SAT II Chemistry Resources
Example Questions
Example Question #1 :Gas Laws
年代uppose that an ideal gas at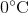

Possible Answers:
正确答案:
Explanation:
First of all, the unit degrees Celsius should be converted to Kelvins by adding 273. This means that the temperature increases from 273 K to 323 K.
Next, the combined gas law can be used.
Plugging in the known values gives
and solving for the volume gives
Rearranging shows this is clearly equivalent to the correct answer,
Gregory
Certified Tutor
Certified Tutor
Pennsylvania State University-Main Campus, Bachelor of Science, Chemical Engineering. Carnegie Mellon University, Doctor of P...
All SAT II Chemistry Resources
Popular Subjects
GMAT Tutors in Seattle,Math Tutors in Houston,MCAT Tutors in Chicago,Chemistry Tutors in Dallas Fort Worth,ACT Tutors in Los Angeles,Algebra Tutors in Houston,Chemistry Tutors in Houston,Calculus Tutors in Denver,Reading Tutors in Phoenix,年代年代AT Tutors in Los Angeles
Popular Courses & Classes
GMAT Courses & Classes in New York City,年代年代AT Courses & Classes in Phoenix,年代panish Courses & Classes in Miami,GMAT Courses & Classes in San Diego,ISEE Courses & Classes in Boston,ACT Courses & Classes in San Francisco-Bay Area,LSAT Courses & Classes in Denver,ISEE Courses & Classes in Philadelphia,年代panish Courses & Classes in San Diego,课程与课程在丹佛
Popular Test Prep
年代AT Test Prep in San Francisco-Bay Area,GMAT Test Prep in San Diego,年代年代AT Test Prep in Chicago,GMAT Test Prep in New York City,ACT Test Prep in San Francisco-Bay Area,MCAT Test Prep in Seattle,年代年代AT Test Prep in Dallas Fort Worth,年代年代AT Test Prep in Washington DC,ACT Test Prep in Philadelphia,年代AT Test Prep in Miami