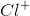All SAT II Chemistry Resources
Example Questions
Example Question #1 :Periodic Trends
The following is a modified true/false question. In it, you must decide if each individual statement is true (T) or false (F). If both are true, then you must also decide if the second statement is a correct explanation (CE) of the first statement.
I: Atoms in the alkaline earth metal group have smaller ionic radii than their atomic radii
BECAUSE
II: atoms in this group usually lose electrons in order to become ions, resulting in the ion lacking the outermost energy level present in the atom.
F, T
T, F
F, F
T, T, CE
T, T (II is not a correct explanation of I)
T, T, CE
The alkaline earth metals are the elements in group II, such as beryllium and magnesium. They want to have a complete octet in their outermost energy level, so they tend to lose two electrons to form the ionic state, which gives them the electron configuration of the noble gas in the previous energy level and thus a smaller ionic radius. Therefore, both statements are true and the second correctly explains the first.
Example Question #1 :Elements And Atoms
The elements with the smallest atomic radii tend to be found in which part of the periodic table?
The upper right corner
The bottom left corner
The bottom right corner
The upper left corner
The inner transition metals
The upper right corner
Atomic radius tends to increase down a group due to the addition of new energy levels; however, it decreases across a period due to increased shielding and increased nuclear mass. For these reasons, the elements with the smallest atomic radii would be in the upper right corner of the periodic table.
Example Question #1 :Elements And Atoms
An atom of phosphorus has 15 protons, 16 neutrons, and 15 electrons. What is the atom's mass number?
Protons and neutrons both have masses of approximately 1 amu, whereas the mass of an electron is negligible. Therefore, the mass number is equal to the number of protons and neutrons, which is 31.
Example Question #1 :Electron Configuration
Which of the following has the electron configuration of![[Ne]3s^23p^6](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1074018/gif.latex)
The task is to find the atom or ion with its first three energy levels completely filled. Argon is not an answer choice, which means that it must be one of the ions with an atomic number close to argon's atomic number. Recalling that a positive charge means electrons were lost from the outermost shell and that a negative charge means electrons were gained in the outermost shell, the only answer that works is
Example Question #2 :Elements And Atoms
Which of the following variables identifies the sublevel that has three orbitals?
The




Certified Tutor
Certified Tutor
All SAT II Chemistry Resources