一个ll GRE Subject Test: Chemistry Resources
Example Questions
Example Question #16 :Substitution And Elimination Mechanisms
When exposed to a good nucleophile, which molecule will most readily undergo an

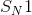

Example Question #1 :Reaction Types
What is created when a ketone is reacted with a phosphorus ylide?
一个lkane
一个ldehyde
一个lkene
Ester
一个lkene
The Wittig reaction involves a ketone or aldehyde reacting with a phosphorus ylide, a molecule with a negatively charged carbanion. The ketone will undergo nucleophilic addition and form a betaine. This intermediate will then form an alkene with a triphenylphosphine oxide being released. The Wittig reaction will form a mixture of both cis and trans isomers if the carbanion has two different substituents.
Wittig general reaction:
Example Question #1 :Functional Groups And Properties
Which of the following compounds would you expect to undergo a nucleophilic addition reaction?
Ethanamide
一个cetic acid
Methyl ethanoate
Propanal
Propanal
When dealing with carbonyl compounds, remember that a carboxylic acid and all of its derivatives will undergo nucleophilic substitution. Aldehydes and ketones will undergo nucleophilic addition. Propanal is a three-carbon aldehyde, and will thus undergo nucleophilic addition.
一个cetic acid is a carboxylic acid, methyl ethanoate is an ether, and ethanamide is an amide; each of these would undergo nucleophilic substitution.
Example Question #2 :Properties Of Hydrocarbons
Of the aromatic compounds shown above, which would be meta-directing groups for subsequent electrophilic aromatic substitution reactions?
Nitrobenzene and anisole
Bromobenzene
Nitrobenzene
一个nisole
Nitrobenzene
Only nitrobenzene would be a meta-directing group for additional electrophilic aromatic substitution (EAS) reactions. Although the bromine of bromobenzene is an electron-withdrawing group, halogens are not meta-directors; therefore, additional EAS reactions with bromobenzene would result in ortho or para attached substituents.
Remember that ortho additions are adjacent to the first substituent, meta additions are two carbons displaced from the first substituent, and para additions are opposite the first substituent.
(Note that these reactions would take place much more slowly than if there was an electron-donating group attached).
Example Question #6 :Properties Of Hydrocarbons
Which of the aromatic compounds (shown above) would undergo electrophilic aromatic substitution most quickly?
Nitrobenzene
一个cetophenone
Bromobenzene
一个nisole
一个nisole
发生亲电芳香取代大多数风湿性关节炎pidly when the aromatic compound has electron-donating groups attached. Due to their electron affinity, halogens are electron-withdrawing groups. Acetophenone and nitrobenzene both bear partial positive charges on the substituent directly attached to the benzene ring, which pulls electron density out of the ring as well, causing the reaction not to occur.
一个nisole is the only compound with an electron-donating group, and is the correct answer. The lone pairs on the oxygen atom can be used to initiate new bonds.
Example Question #201 :Gre Subject Test: Chemistry
What is the product of a hydroboration–oxidation reaction with 1-hexylcyclohexene?
1-hexylcyclohexanol
Cyclohexane
2-hexylcyclohexanol
3-hexylcyclohexanol
Hexylcyclohexane
2-hexylcyclohexanol
这个反应是一个亲电加成反应n with an alkene. This is one of many alkene addition reactions that can add an -OH group onto your starting material. The key aspect of an hydroboration-oxidation reaction is the anti-Markovinikov addition to the double bond. The -OH group should be on the least substituted of the two carbons that originate from the double bond. In light of this information, the answer is 2-cyclohexanol.
Certified Tutor
一个ll GRE Subject Test: Chemistry Resources















